
A Phase 2/3 Open-Label Study of ELA026 in Treatment-Naïve Adult and Pediatric Participants With Secondary Hemophagocytic Lymphohistiocytosis (sHLH)

Key Objectives of the SURPASS Study
The SURPASS study will assess the safety, efficacy, pharmacokinetics, and pharmacodynamics of ELA026 in participants with secondary Hemophagocytic Lymphohistiocytosis (sHLH), a rare, life-threatening condition marked by excessive immune activation.1
ELA026 is a first-in-class monoclonal antibody that binds to cells expressing signal regulatory protein (SIRP)-α, -β1, and -γ, and targets these cells for depletion. SIRP-expressing cells are believed to drive the hyperinflammatory response that is characteristic of sHLH.
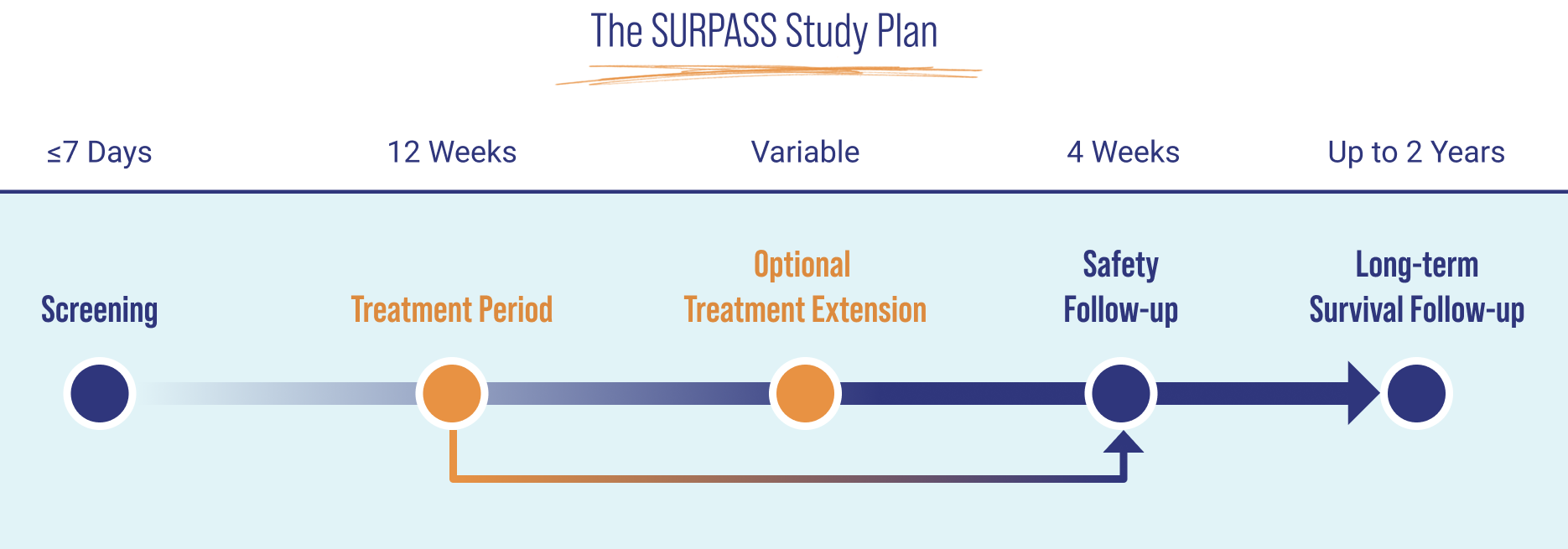
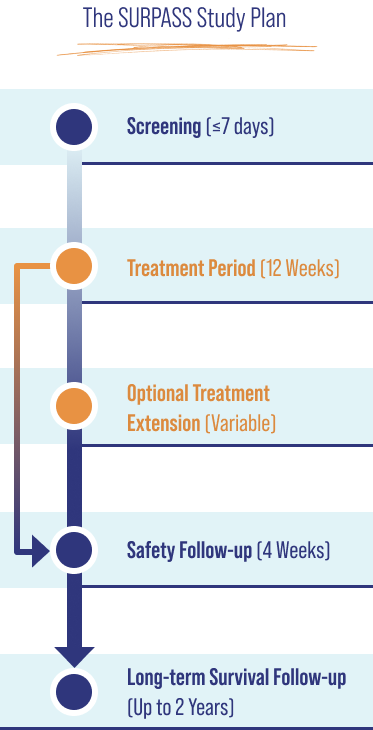

ELA026 is formulated for intravenous (IV) or subcutaneous (SC) injection.
| Study Day | Dose | Dosing Method |
|---|---|---|
| 1 | 0.1 mg/kg | IV |
| 2 | 0.3 mg/kg | IV |
| 3 | 0.3 mg/kg | IV |
| 4 | 0.3 mg/kg | IV |
| 2x per week starting at Day 8 | 0.5 mg/kg | IV or SC |
Reference: 1. Konkol S, Killeen RB, Rai M. Hemophagocytic Lymphohistiocytosis. [Updated 2025 May 3]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2025 Jan. https://www.ncbi.nlm.nih.gov/books/NBK557776/

Key Inclusion Criteria
Cohort A
 |
Adults with treatment-naïve, malignancy-associated HLH |
Cohort B
 |
Adults with treatment-naïve sHLH not triggered by malignancy |
 |
Adults with treatment-naïve, malignancy-triggered HLH, diagnosed by Optimized HLH Inflammatory (OHI) Index1 |
 |
13- to 17-year-olds with treatment-naïve sHLHa |
 |
6- to 12-year-olds with refractory sHLHa (safety lead-in cohort) |
 |
6- to 12-year-olds with treatment-naïve sHLHa (after completion of safety lead-in cohort) |
Diagnosis of HLH will be confirmed by participants meeting five of eight HLH-2004 criteria, except where noted.
aEnrollment of patients <15 years old will be restricted to specific countries.
Reference: 1. Zoref-Lorenz A, Murakami J, Hofstetter L, et al. An improved index for diagnosis and mortality prediction in malignancy-associated hemophagocytic lymphohistiocytosis. Blood. 2022;139(7):1098-1110. doi:10.1182/blood.2021012764
Key Exclusion Criteria

|
Refractory sHLH (except for safety lead-in cohort) |

|
Known or suspected primary or hereditary HLH |

|
Severe organ dysfunction |

|
Any other significant concurrent, uncontrolled medical condition that contraindicates participation in this study or prohibits completion of study procedures |

|
End-stage malignancy for which no suitable therapies are available to treat the malignancy triggering the HLH |

|
Allogeneic hematopoietic stem cell transplant within 100 days prior to the first dose of ELA026 |
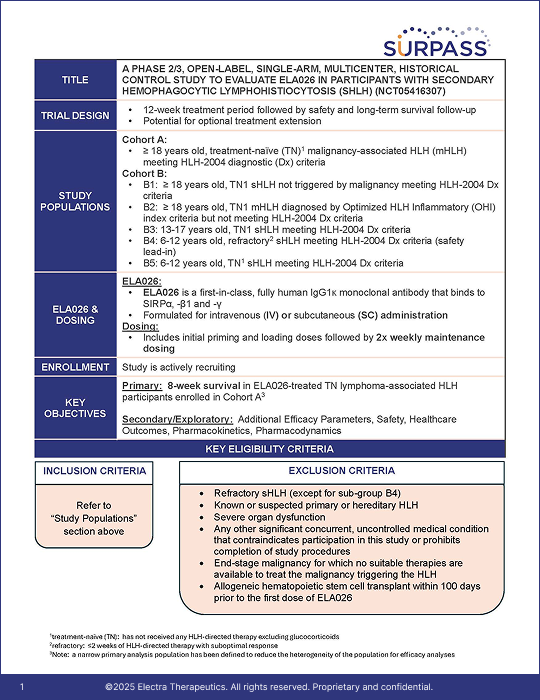
Clinical Trial Summary
SURPASS is a Phase 2/3, open-label, single-arm, multicenter, historical control study that evaluates ELA026 in adult and pediatric patients aged ≥6 years with sHLH.a The primary objective is to assess the effect of ELA026 on 8-week survival in treatment-naïve lymphoma-associated HLH participants.
Secondary objectives include other efficacy parameters, safety, healthcare outcomes, pharmacokinetics, and pharmacodynamics.
Download the SURPASS study one-page summary below.

© 2025 Electra Therapeutics Inc.
No Contact Available
Site has not yet provided contact information. You may wish to check back again in the future.
Please complete the challenge
There is no contact information available for this site at this time. Please check back later.
You are leaving our site and visiting an external resource (ClinicalTrials.gov). We do not own or operate this website.
The page will open automatically in 10 seconds.
"*" indicates required fields
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

