ELA026: Targeted Myeloid Cell and T Lymphocyte Depletor
ELA026 is a monoclonal antibody specifically designed to address severe inflammatory disorders with aberrant myeloid cell and T lymphocyte activity. It induces rapid, potent, and targeted depletion of the circulating myeloid cells and T lymphocytes that drive inflammation, without disrupting the CD47/SIRPα immune checkpoint function.

Treating sHLH, Offering Hope
There are no approved therapies for sHLH. Current off-label treatments for sHLH carry significant long-term toxicity and limited efficacy.3,4 Even with these treatments, sHLH has high mortality during the first few months.5 Malignancy-associated HLH (mHLH) is the deadliest amongst the subtypes of sHLH, with a mortality rate of approximately 50% at 2 months.6
ELA026 Targets the Principal Cells Responsible for Driving Pathogenesis in sHLH

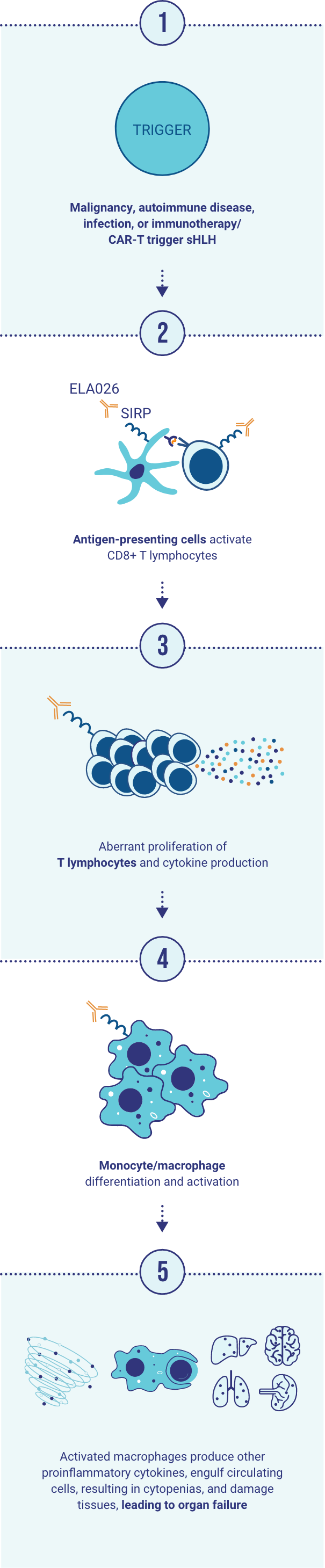
By targeting SIRP on the cell surface of myeloid cells and T lymphocytes, ELA026 depletes the cells responsible for inducing the hyperinflammatory condition in sHLH.
By targeting SIRP on the cell surface of myeloid cells and T lymphocytes, ELA026 depletes the cells responsible for inducing the hyperinflammatory condition in sHLH.
CAR-T=chimeric antigen receptor (CAR)-T cell.
References: 1. Otrock Z, Eby C. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2015;90(3):220-224. doi:10.1002/ajh.23911 2. Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577-4584. doi:10.1182/blood-2011-06-356261 3. Bergsten E, Horne A, Arico M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349 4. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618 5. Abdelhay A, Mahmoud A, Mostafa M, et al. Delay in treatment of adult hemophagocytic lymphohistiocytosis is associated with worse in-hospital outcomes. Ann Hematol. 2023;102(11):2989-2996. doi:10.1007/s00277-023-05271-w 6. Lionel AC, Long JP, Prakash R, et al. Differing Inflammatory Profiles and Survival Outcomes of Cytokine Storm Arising from CAR T-Cell Therapy, Covid, and Malignancy-Associated HLH in Patients with Hematological Malignancies. Poster presented at the American Society of Hematology 2023. Poster: SUN-3507.
No Contact Available
Site has not yet provided contact information. You may wish to check back again in the future.
There is no contact information available for this site at this time. Please check back later.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |